How to Calculate pH: Effective Ways to Understand the Basics for Accurate Results
Understanding the pH Scale Definition
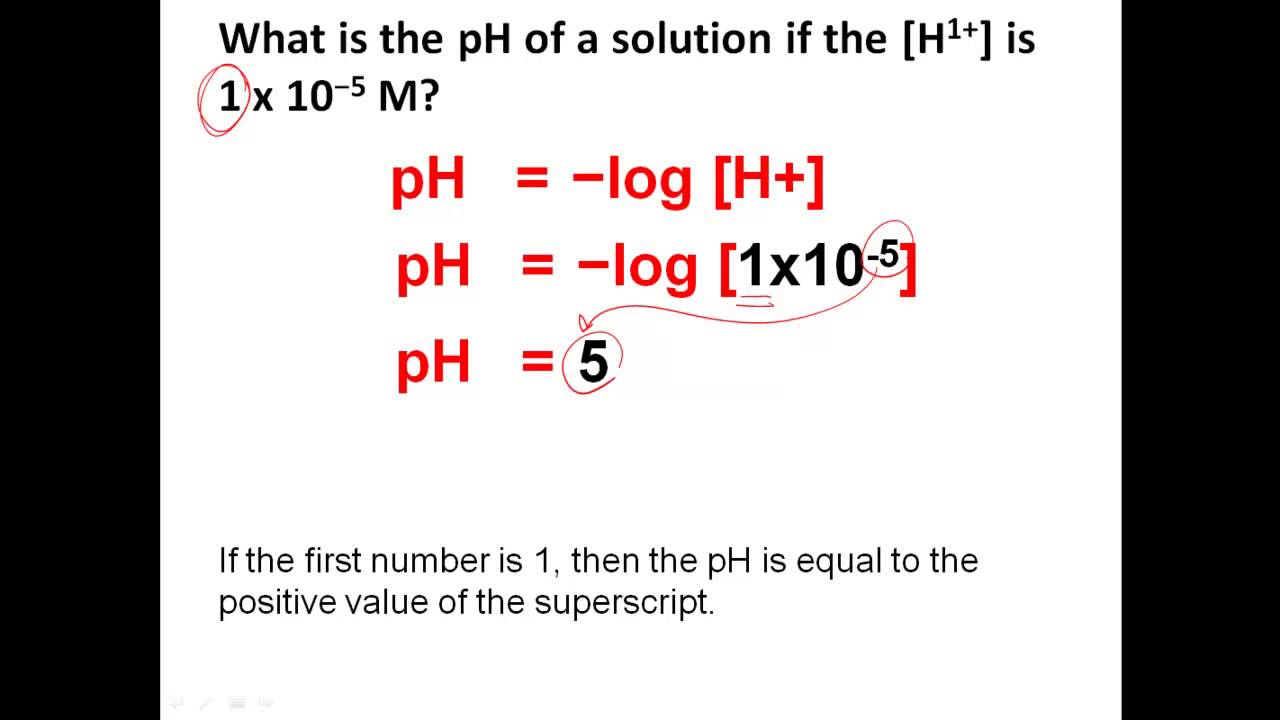
The **pH scale** is a widely used measurement tool that indicates the acidity or alkalinity of a solution. Operating on a scale from 0 to 14, where 7 represents a neutral state, values lower than 7 signify acidity and those higher indicate alkalinity. **Measuring pH levels** is crucial in various fields, including chemistry, biology, agriculture, and environmental science, as it affects chemical reactions, microbial activity, and overall ecosystem health. Understanding the **importance of pH** lies in its ability to inform how substances interact, making it accessible for adjustments and manipulations in real-world applications. Various **instruments for pH measurement**, such as pH meters and pH test strips, facilitate this essential measurement, ensuring environments are maintained at optimal baselines for varied processes and functions.
The Impact of Temperature on pH
Temperature significantly influences the **pH of solutions**. As temperature changes, the behavior of ions in solution alters, potentially affecting acidity and alkalinity measurements. Higher temperatures generally lead to reduced pH levels, as the dissociation of water increases, producing more hydrogen ions. It’s essential to adjust readings to account for temperature differences, primarily if your measurements occur in environments outside controlled conditions, like **pH in swimming pools** or **pH in agriculture** where ambient temperatures fluctuate significantly. For accurate **pH calculation methods**, it’s advisable to calibrate instruments and reference data accordingly, ensuring you achieve reliable and reproducible results.
Standard pH Values
Familiarizing yourself with common **standard pH values** can ease the interpretation of pH readings. For example, pure water sits at a pH of 7, while lemon juice, a common dietary staple, registers around 2, indicating its strong acidic nature. Moreover, the **pH in food science** often targets optimal ranges for fermentation, preservation, and overall taste enhancement. Each substance plays a unique role in its respective application; hence, having a robust understanding of these values is crucial for professionals in food processing, pharmaceuticals, and environmental monitoring. Regular calibration of measurement tools ensures consistency in these readings, sustaining quality in outputs.
Measuring pH Levels: Techniques and Equipment
Accurate pH measurement relies significantly on the chosen technique and equipment. Two primary tools utilized are **pH meters** and **pH test strips**. Each comes with advantages and particular use cases. **pH meters** offer precise digital readings, making them ideal for laboratory setups and industrial applications. In contrast, **pH test strips** are portable, cost-effective, and easy to use, which suits fieldwork, initial screenings, and educational purposes. Familiarity with the specifications of each tool empowers users to make informed choices based on their specific **pH calculation method** needs, enhancing the accuracy of their results regardless of the context, be it in agriculture or water quality analysis.
Pioneering pH Meter Usage
Utilizing a **pH meter usage** starts with understanding the calibration procedures. A standard protocol involves immersing the electrode in calibrated buffer solutions with known pH values to align readings appropriately. This step mitigates errors, ensuring accurate measurements across varying conditions. For practitioners in **pH management in aquariums** or stringent laboratory research, following these calibration methods is fundamental. Maintaining your pH meter’s cleanliness and storing it correctly also ensures longevity and consistency in performance, which is paramount in environments where precise measurements hold considerable importance.
Implementing pH Test Strips Effectively
**pH test strips** provide a straightforward alternative for measuring pH levels with minimal equipment. Users dip the strip into the solution, which changes color to correspond with a pH value range marked on its packaging. While less accurate than pH meters, they are an excellent choice for quick assessments, such as testing **pH in biology** experiments or evaluating soil pH for agricultural applications. It’s vital to compare colors in natural light for the most reliable comparison outcomes, ensuring valid interpretations and suitable actions taken based on varying **pH levels in different liquids**.
Applications of pH Measurement: From Agriculture to Pharmaceuticals
The influence of pH levels spans a variety of fields beyond basic chemistry. In agriculture, for instance, **pH in soil testing** determines nutrient availability and vegetative growth potential. Many plants thrive within specific pH ranges, and **pH management in agriculture** is essential for maximizing crop yields and health. Meanwhile, in pharmaceuticals, formulations require stringent pH control to ensure drug efficacy and stability. Understanding the significance of these applications illustrates pH’s pivotal role in chemical properties, environmental stability, production quality, and biological processes across diverse sectors. Moreover, knowledge of **pH in fermentation** aids brewers and in food product formulation, emphasizing its value in product development.
pH in Food Science
Understanding **pH in food science** is critical for food safety and preservation. Many food items must be processed at specific pH levels to inhibit microbial growth, extend shelf-life, and enhance flavors. For instance, fermentation in processes like yogurt or sauerkraut production heavily relies on maintaining the right acidity levels for active cultures. This not only preserves the product but significantly impacts the food’s final taste and texture. It is vital for food manufacturers to consistently measure pH, utilizing appropriate instruments that yield accurate results. Moreover, igniting a collective understanding of the **importance of pH in winemaking** showcases how acidity balances sweetness, creating delightful beverage profiles.
The Role of pH in Water Quality
Maintaining optimal **pH and water quality** is essential for ecosystems in both natural and controlled environments. For example, in aquatic systems, variations in water pH can significantly influence species composition and ecosystem health. Regular monitoring aids in revealing anomalies tied to pollution or other environmental stresses. Understanding the **pH implications in wastewater treatment** illustrates the necessity of managing interactions that ensure decomposing materials break down appropriately without harmful repercussions. The interconnections highlighted here exemplify the wide-reaching importance of pH testing in sustaining balanced ecosystems and microbial health while ensuring regulatory compliance is met.
Key Takeaways
- The **pH scale** ranges from 0 to 14 and indicates acidity or alkalinity.
- Temperature impacts pH measurements, necessitating calibration adjustments.
- Tools like **pH meters** and **pH test strips** offer various levels of accuracy for measuring pH levels.
- **pH in agriculture**, pharmaceuticals, and food science illustrate its pervasive importance across industries.
- Understanding pH influences water quality and ecosystem health, emphasizing its relevance in environmental science.
FAQ
1. What is the significance of measuring pH in agriculture?
Measuring pH in agriculture is crucial for determining soil health and nutrient availability. Most plants thrive within specific pH ranges, and understanding these values helps farmers optimize growth conditions. Adjusting soil pH can enhance nutrient uptake, leading to increased crop yield and overall plant vitality. This practice is a fundamental aspect of efficient agricultural management.
2. How does temperature affect pH readings?
Temperature can significantly affect pH readings by and affecting ion activity in solutions. Higher temperatures usually lead to lower pH values, indicating a shift toward increased acidity. This is especially influential when assessing conditions in environments like **pH in swimming pools**, where fluctuations can lead to discomfort or require chemical adjustments.
3. What are effective ways to adjust pH levels?
Effective methods to adjust pH levels include adding **buffer solutions** that help stabilize the pH, applying amendments such as lime to raise pH, or utilizing sulfur to decrease it. In laboratory testing, tailored chemicals may also be employed to achieve desired acidity or alkalinity levels based on specific application needs across fields, including **pH in pharmaceuticals** and food science.
4. What instruments are best for measuring pH in food production?
In food production, both **pH meters** and **pH test strips** are used. pH meters offer precise readings necessary for strict formulations, whereas test strips provide a cost-effective alternative for quick checks. Each instrument suits different needs, allowing manufacturers to ensure product safety and quality.
5. How do common household items demonstrate pH variations?
Common household items exhibit various pH levels, such as lemon juice (around 2), vinegar (around 3), and baking soda (around 9). Understanding these variations can inform safe use and pH adjustments in food preparation, cleaning applications, or gardening practices. This basic knowledge aids in regular interfacing with these substances safely and effectively.
6. How do pH test strips compare to pH meters?
**pH test strips** are simpler and cheaper but may not provide the precision of **pH meters**. The strips function through color change, while meters use electronic sensors for direct readings. While test strips suit casual or initial assessments, meters are ideal for in-depth laboratory analyses or when exact measurements are critical.
7. Why is pH important in environmental science?
pH is crucial in environmental science because it influences nutrient availability, chemical reactions, and microbial activities in ecosystems. Monitoring pH can indicate the health of natural systems, detect pollution, and prioritize conservation efforts. Understanding these parameters helps address broader environmental issues.