How to Properly Name Ionic Compounds
Understanding Ionic Compounds and Their Naming Rules
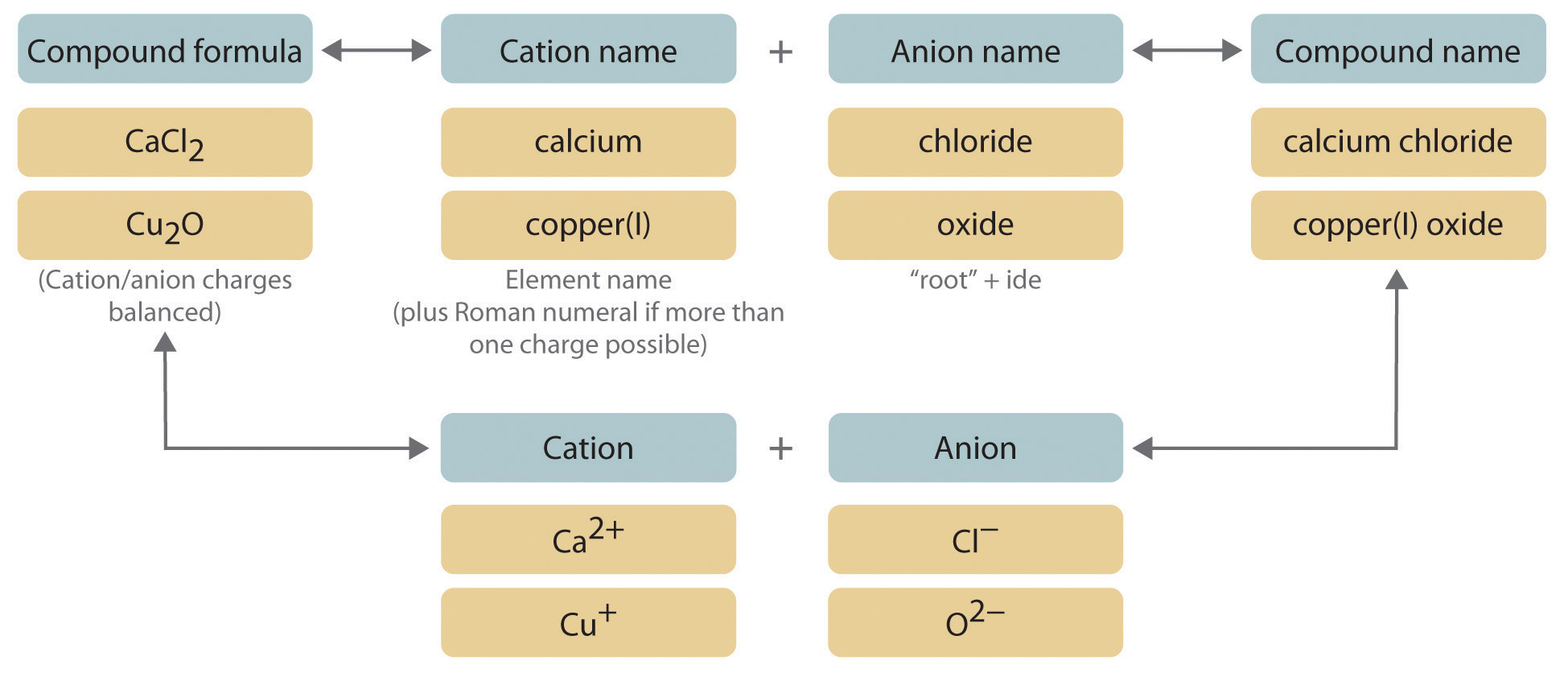
Ionic compounds are essential in chemistry, characterized by the transfer of electrons from metals to nonmetals, resulting in the formation of charged ions. Understanding how to name ionic compounds correctly is crucial for effective communication in the scientific community. The ionic compound naming rules lay the foundation for identifying these substances. This involves naming the positive ion (cation) first, followed by the negative ion (anion). Moreover, it’s important to use correct nomenclature to reflect the composition and oxidation states of the ions involved. By mastering these fundamental rules, one can successfully engage in chemical discussions and practical applications related to ionic compounds.
Naming Based on Charge: Key Factors to Consider
The principle of naming ionic compounds based on charge is vital. Each ion carries a specific charge, which can often be determined from the element’s position in the periodic table. For instance, alkali metals always form cations with a +1 charge, while alkaline earth metals form cations with a +2 charge. Nonmetals typically form anions with a negative charge corresponding to the number of additional electrons needed to achieve a full valence shell. Using these fundamentals, you can determine the cation and anion names accurately. For example, in sodium chloride (NaCl), sodium (Na+) is the cation with a +1 charge, while chloride (Cl-) is the anion with a -1 charge.
Using Oxidation States in Naming Ionic Compounds
The concept of oxidation states plays a significant role, particularly when dealing with transition metals, which can exhibit multiple oxidation states. For instance, when naming compounds like iron(III) oxide (Fe2O3), the Roman numeral indicates the oxidation state of the iron ion. Familiarizing yourself with oxidation states in naming allows for precise communication of ionic compound identities. Furthermore, for compounds containing polyatomic ions, the names of these ions must be recognized and accurately integrated into the compound name, maintaining clarity and reducing confusion.
Common Mistakes in Ionic Naming
Understanding common mistakes in ionic naming is crucial for mastering this topic. A frequent error occurs when students forget to consider the charge of transition metals and neglect to add appropriate oxidation state indicators. Additionally, confusing similar sounding polyatomic ion names can lead to incorrect compound identifications. To avoid these pitfalls, using a reliable worksheet for ionic compounds can enhance learning and retention. Practice and repetitive exposure to naming conventions help solidify these concepts, ensuring successful mastery of the topic.
Nomenclature for Ionic Compounds: From Basics to Advanced Techniques
In chemistry, the nomenclature of ionic compounds varies between binary compounds and those containing polyatomic ions. A firm grasp of these differences is critical for accurate chemical communication. Additionally, incorporating traditional names for ionic compounds alongside systematic names can aid in understanding. Some ionic compounds, such as NH4Cl (ammonium chloride), require nuanced approaches to naming protocols, especially when dealing with mixtures of ionic compounds.
Basic Ionic Naming Rules
The basic ionic naming rules stipulate that the cation’s name remains unchanged, while the anion name typically ends with an ‘-ide’ suffix for monatomic ions. For example, sodium bromide (NaBr) illustrates this rule well. When dealing with polyatomic ions, names are derived from their specific characteristics, such as sulfate for SO4^2- or nitrate for NO3^-. Recognizing these distinctions between polyatomic and monatomic ions is essential in ensuring proper naming techniques across diverse ionic compounds.
Naming Ionic Compounds with Polyatomic Ions
When naming ionic compounds that include polyatomic ions, understanding these ion structures and names is crucial. Common examples include ammonium (NH4^+) and carbonate (CO3^2-). When these ions combine with other cations, such as calcium, the compound formed is calcium carbonate (CaCO3). Knowing how to combine the cation name with the appropriate polyatomic ion assistance ensures accuracy in chemical nomenclature and supports students in identifying and understanding ionic compounds in chemistry.
Expert Tips for Effective Naming of Ionic Compounds
There are some expert tips on naming ionic compounds that can significantly enhance mastery. Utilizing mnemonics can help remember complex nomenclature rules, especially for polyatomic ions. Engaging in interactive tools for naming can also amplify understanding while addressing diverse learning styles and preferences. Additionally, practicing through quizzes and worksheets regularly facilitates optimal learning and helps solidify the correct usage of names and formulas associated with ionic compounds.
Practical Applications & Importance of Proper Naming
The importance of accurate ionic naming extends to many daily activities and professional maneuvers. Correct naming practices not only clarify communication within the scientific community but also ensure precision in various fields such as pharmaceuticals and material sciences. Recognizing common ionic compounds is equally essential, as substances like table salt (NaCl) or baking soda (NaHCO3) are fundamental components in numerous applications.
Common Ionic Compounds List
Familiarity with a common ionic compounds list greatly benefits those studying chemistry. Compounds such as potassium chloride (KCl), magnesium sulfate (MgSO4), and aluminum oxide (Al2O3) frequently appear in educational materials and can serve as benchmarks for understanding ionic compound structures. Learning these examples enables students and professionals to become adept at quickly identifying and engaging with various ionic compounds.
Ionic Names in Daily Applications
Ionic compounds are prevalent in our daily lives, from food to cleaning products. Understanding which compounds are ionic allows consumers to make informed choices about their use. For instance, recognizing that chemical fertilizers often contain ammonium nitrate shows the practical application of ionic naming knowledge. Engaging students and consumers in discussions about these real-world applications highlights the significance of naming conventions in chemistry.
Key Takeaways
- Understanding cations and anions is crucial for naming ionic compounds.
- Mastering oxidation states greatly improves the accuracy of compound names.
- Polyatomic ions require recognition of their specific names for proper naming.
- Utilizing worksheets and practice is vital for learning appropriate practices.
- Common ionic compounds exist in our daily lives, making naming knowledge practical and applicable.
FAQ
1. What is the importance of ionic naming in chemistry?
The importance of ionic naming lies in its ability to facilitate clear communication among scientists. Proper nomenclature ensures that chemical substances are correctly identified and understood, preventing errors in communication that could lead to dangerous misinterpretations in various fields, including pharmacology and chemical manufacturing.
2. Can you explain the difference between traditional and systematic naming?
Traditional naming involves using established, historical names of compounds, while systematic naming refers to the structured approach defined by IUPAC rules. For example, FeO is traditionally known as ferrous oxide, whereas its systematic name is iron(II) oxide, clarifying the oxidation state of the metal involved.
3. How can I improve my understanding of ionic nomenclature?
Improving understanding involves consistent practice, utilizing worksheets and quizzes tailored to naming ionic compounds, and exploring interactive resources. Engaging in group discussions or tutoring can also facilitate deeper comprehension. Solidifying foundational principles makes complex naming more manageable and cohesive.
4. What is a common mistake students make in ionic naming?
One common mistake is neglecting to account for the oxidation states of transition metals, causing incorrect naming or formula writing. To avoid this, always remember to include Roman numerals to indicate the oxidation state when naming transition metal compounds.
5. How do polyatomic ions affect ionic compound naming?
Polyatomic ions contribute complexity to naming ionic compounds as they have specific names that differ from their elemental components. Understanding these names and how they combine with cations is essential for accurately naming compounds containing them, like sodium sulfate (Na2SO4).
6. Are there examples of ionic compounds used in everyday life?
Yes, ionic compounds are commonly found in everyday products. For instance, table salt (sodium chloride) is an ionic compound used in cooking, while magnesium sulfate can be found in bath salts. Familiarizing yourself with these examples enhances understanding of where ionic naming applies contextually.